Tokyo, Japan

Medical institutions that provide regenerative medicine using stem cells
create a regenerative medicine provision plan for each regenerative medicine technology they implement, and have it reviewed for safety, effectiveness, etc.
by a specified certified regenerative medicine committee approved by the Ministry of Health, Labor and Welfare receive.
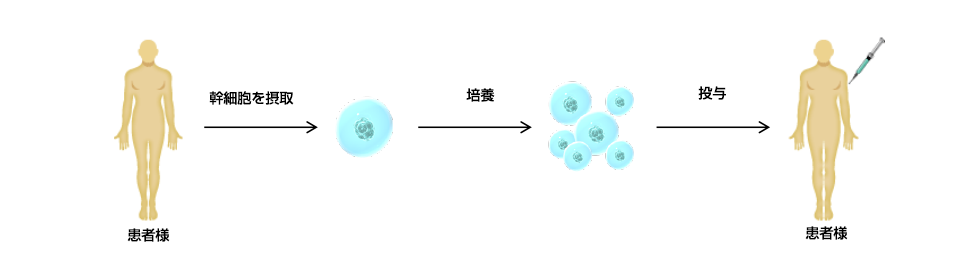
幹細胞を用いる再生医療等を提供する医療機関は、
実施する再生医療技術ごとに再生医療等提供計画を作成し、
厚生労働省が認可した特定認定再生医療等委員会によって
安全性、有効性等について審査を受けます。
Aging care (intravenous therapy/injection)
Various stem cell treatments such as anti-aging prevention
エイジングケア(点滴療法・注射)
抗齢予防などの様々な治療
All of our staff members are committed to working closely with you and providing medical care to the best of our ability. We look forward to serving you, with the promise of advanced workmanship and high-quality hospitality.
皆様に寄り添い、スタッフ一同 全力で診療にあたる所存でございます。
高度な技量と上質のホスピタリティをお約束し、心よりお待ち申し上げます。
Potential of mesenchymal stem cells
There are approximately 60 trillion cells in our body. Our tissues, such as the heart, brain, and skin, are made up of cells that play an extremely important role in our daily lives. There are approximately 270 types of cells depending on their role. The cells that form the basis of these cells are “stem cells.” Stem cells are cells that transform into various cells and tissues and exist in fixed locations in the body. These cells produce cells to replace tissue when it is damaged.
It has long been known that these stem cells exist in the bone marrow. However, there is a limit to the amount of stem cells that can be harvested from bone marrow, and harvesting requires general anesthesia, which is a burdensome operation for patients. In 2000, Dr. Hedrick of UCLA (University of California, Los Angeles) discovered that abdominal subcutaneous fat contains a large amount of stem cells. Various studies have been conducted on the basic characteristics of adipose-derived mesenchymal stem cells. Adipose-derived mesenchymal stem cells, which are easy to collect, highly safe, and have a wide range of efficacy, are attracting attention as cells that are key to regenerative medicine.
In addition, the “Act on Ensuring the Safety of Regenerative Medicine, etc. (Act No. 85 of 2013)” was enacted on November 25, 2014, and clinical research (clinical research using human stem cells, etc.) is free. Rules to be respected by medical institutions and cell processing product manufacturers have been established so that research, development, and practical application of high-quality regenerative medicine can proceed safely in medical practice, and in order to provide regenerative medicine quickly and safely. The environment is now in place.
間葉系幹細胞の可能性~
私たちの身体の中には約60兆個の細胞が存在します。心臓、脳、皮膚など私たちの組織は細胞が集まってできており、日常で非常に重要な役割を果たしています。 役割によって約270種類の細胞に分けられます。それらの細胞の元となる細胞が『幹細胞』です。幹細胞はいろいろな細胞や組織に変化する細胞で、体内の決まった場所に存在します。組織が損傷した場合などにその組織を補うための細胞を生み出す細胞です。
この幹細胞は以前から骨髄に存在する事が知られていました。しかし骨髄から採取できる幹細胞には限りがあり、採取する際は全身麻酔が必要になるなど患者様に負担のかかる手術が必要でした。2000年にUCLA(カリフォルニア 大学ロサンゼルス校)のDr.Hedrickが腹部皮下脂肪に大量の幹細胞が含まれている事を発見しました。脂肪由来間葉 系幹細胞の基本的な特性については、様々な研究が積み重ねられています。採取が容易で、安全性が高く、その有効性が多岐にわたる脂肪由来間葉系幹細胞は、再生医療の鍵となる細胞として注目を集めています。
また、「再生医療等の安全性の確保等に関する法律(平成25年法律第85号)」が平成26年11月25日に施行され、臨床研究(ヒト幹細胞を用いた臨床研究等)、自由診療のもとで良質な再生医療等の研究開発や実用化が安全に進められるよう医療機関や細胞加工物製造事業者が尊重するルールが定められ、再生医療等の迅速かつ安全な提供するための環境が整ってきています。
![]() Information Inquiry via WhatsApp :
Information Inquiry via WhatsApp :
e-mail: [email protected]
Disclaimer: Stem cell therapy is a great alternative therapy but offers no guarantees and is not promoted as a cure. This is similar to many other conventional medical treatments. A complete review of the patient’s medical history is required to determine eligibility and approval for stem cell therapy. All personal information provided is for internal and medical use with medical providers only. Treatments are not conducted in offices or in the UK. All cellular therapies are conducted in Europe or Japan, as it is regulated by the Ministry of Health and other local authorities. All treatments are performed within the legal limits and regulatory framework for the country in which the specific medical provider practices.







